Copper sulfate is very easy to obtain in large quantities at gardening and hardware stores and provides a convenient route to sulfuric acid if the appropriate anode can be obtained. WebDuring an electrolysis of conc. Of course, the water molecules are present in the highest concentration, much higher than the other species, since it is a dilute solution. Is it Possible to Neutralize Sulfuric Acid With Water? WebElectrolysis of concentrated sulphuric acid In my textbook it is given that for electrolysis of dilute sulfuric acid at anode following reactions can occur: At moderate concentrations 2 H A 2 O O A 2 + H A + + 4 e A And for high concentrations 2 For the production or manufacture of sulfuric acid, the material required is dry and clean sulfur dioxide gas. WebIn one of its most familiar applications, sulfuric acid serves as the electrolyte in lead acid storage batteries. They are precursors of different components, for example, H. S, taurine, sulfates, glutathione and work on oxidative status and various signalling pathways. Then, the water will dilute and carry off some amount of heat that either the bicarbonate or sodium carbonate produces when it neutralizes the acid. Sulfuric acid is also present in samples of gas for CEMS. The concentrated sulfuric acid breaks down In the phenol sulfuric acid method into any polysaccharides, oligosaccharides, and disaccharides into monosaccharides. WebSulfuric acid electrolysis process wherein; a temperature of electrolyte containing sulfuric acid to be supplied to an anode compartment and a cathode compartment is controlled to 30 degree Celsius or more; a flow rate F1 (L/min.) Sulfur trioxide, the anhydride of sulfuric acid, is the immediate precursor. This is formed through the oxidation of elemental sulfur. In such Electrolysis 2.24 L of H2 amd 0.56 L O2 were produced at STP. Keep the boiling tube in water for around three hours with 5mL of 2.5 N-HCl in order to hydrolyse it, then cool it to room temperature. Electrolysis of concentrated sulfuric acid, During the electrolysis of concentrated sulfuric acid, the hydrogen ions (H. ) move into the cathode and are discharged. WebIn one of its most familiar applications, sulfuric acid serves as the electrolyte in lead acid storage batteries. 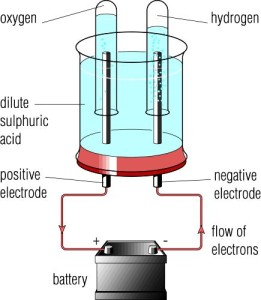 WebIn this video, I show how to make concentrated sulfuric acid at home. It has one atom of sulfur, four oxygen atoms attached to the sulfur atom and two hydrogen atoms attached with two oxygen atoms. Its molecular weight is 98.079 g/mol. gives the following atanode (1) H2 (2) O2 (3) H2S203 (4) H2S2O8, can be prepared by electrolytic oxidation of, NCERT Solutions Class 12 Business Studies, NCERT Solutions Class 12 Accountancy Part 1, NCERT Solutions Class 12 Accountancy Part 2, NCERT Solutions Class 11 Business Studies, NCERT Solutions for Class 10 Social Science, NCERT Solutions for Class 10 Maths Chapter 1, NCERT Solutions for Class 10 Maths Chapter 2, NCERT Solutions for Class 10 Maths Chapter 3, NCERT Solutions for Class 10 Maths Chapter 4, NCERT Solutions for Class 10 Maths Chapter 5, NCERT Solutions for Class 10 Maths Chapter 6, NCERT Solutions for Class 10 Maths Chapter 7, NCERT Solutions for Class 10 Maths Chapter 8, NCERT Solutions for Class 10 Maths Chapter 9, NCERT Solutions for Class 10 Maths Chapter 10, NCERT Solutions for Class 10 Maths Chapter 11, NCERT Solutions for Class 10 Maths Chapter 12, NCERT Solutions for Class 10 Maths Chapter 13, NCERT Solutions for Class 10 Maths Chapter 14, NCERT Solutions for Class 10 Maths Chapter 15, NCERT Solutions for Class 10 Science Chapter 1, NCERT Solutions for Class 10 Science Chapter 2, NCERT Solutions for Class 10 Science Chapter 3, NCERT Solutions for Class 10 Science Chapter 4, NCERT Solutions for Class 10 Science Chapter 5, NCERT Solutions for Class 10 Science Chapter 6, NCERT Solutions for Class 10 Science Chapter 7, NCERT Solutions for Class 10 Science Chapter 8, NCERT Solutions for Class 10 Science Chapter 9, NCERT Solutions for Class 10 Science Chapter 10, NCERT Solutions for Class 10 Science Chapter 11, NCERT Solutions for Class 10 Science Chapter 12, NCERT Solutions for Class 10 Science Chapter 13, NCERT Solutions for Class 10 Science Chapter 14, NCERT Solutions for Class 10 Science Chapter 15, NCERT Solutions for Class 10 Science Chapter 16, NCERT Solutions For Class 9 Social Science, NCERT Solutions For Class 9 Maths Chapter 1, NCERT Solutions For Class 9 Maths Chapter 2, NCERT Solutions For Class 9 Maths Chapter 3, NCERT Solutions For Class 9 Maths Chapter 4, NCERT Solutions For Class 9 Maths Chapter 5, NCERT Solutions For Class 9 Maths Chapter 6, NCERT Solutions For Class 9 Maths Chapter 7, NCERT Solutions For Class 9 Maths Chapter 8, NCERT Solutions For Class 9 Maths Chapter 9, NCERT Solutions For Class 9 Maths Chapter 10, NCERT Solutions For Class 9 Maths Chapter 11, NCERT Solutions For Class 9 Maths Chapter 12, NCERT Solutions For Class 9 Maths Chapter 13, NCERT Solutions For Class 9 Maths Chapter 14, NCERT Solutions For Class 9 Maths Chapter 15, NCERT Solutions for Class 9 Science Chapter 1, NCERT Solutions for Class 9 Science Chapter 2, NCERT Solutions for Class 9 Science Chapter 3, NCERT Solutions for Class 9 Science Chapter 4, NCERT Solutions for Class 9 Science Chapter 5, NCERT Solutions for Class 9 Science Chapter 6, NCERT Solutions for Class 9 Science Chapter 7, NCERT Solutions for Class 9 Science Chapter 8, NCERT Solutions for Class 9 Science Chapter 9, NCERT Solutions for Class 9 Science Chapter 10, NCERT Solutions for Class 9 Science Chapter 11, NCERT Solutions for Class 9 Science Chapter 12, NCERT Solutions for Class 9 Science Chapter 13, NCERT Solutions for Class 9 Science Chapter 14, NCERT Solutions for Class 9 Science Chapter 15, NCERT Solutions for Class 8 Social Science, NCERT Solutions for Class 7 Social Science, NCERT Solutions For Class 6 Social Science, CBSE Previous Year Question Papers Class 10, CBSE Previous Year Question Papers Class 12, JEE Main 2022 Question Paper Live Discussion. Vedantu LIVE Online Master Classes is an incredibly personalized tutoring platform for you, while you are staying at your home. It is one of the most important chemicals from the commercial point of view.
WebIn this video, I show how to make concentrated sulfuric acid at home. It has one atom of sulfur, four oxygen atoms attached to the sulfur atom and two hydrogen atoms attached with two oxygen atoms. Its molecular weight is 98.079 g/mol. gives the following atanode (1) H2 (2) O2 (3) H2S203 (4) H2S2O8, can be prepared by electrolytic oxidation of, NCERT Solutions Class 12 Business Studies, NCERT Solutions Class 12 Accountancy Part 1, NCERT Solutions Class 12 Accountancy Part 2, NCERT Solutions Class 11 Business Studies, NCERT Solutions for Class 10 Social Science, NCERT Solutions for Class 10 Maths Chapter 1, NCERT Solutions for Class 10 Maths Chapter 2, NCERT Solutions for Class 10 Maths Chapter 3, NCERT Solutions for Class 10 Maths Chapter 4, NCERT Solutions for Class 10 Maths Chapter 5, NCERT Solutions for Class 10 Maths Chapter 6, NCERT Solutions for Class 10 Maths Chapter 7, NCERT Solutions for Class 10 Maths Chapter 8, NCERT Solutions for Class 10 Maths Chapter 9, NCERT Solutions for Class 10 Maths Chapter 10, NCERT Solutions for Class 10 Maths Chapter 11, NCERT Solutions for Class 10 Maths Chapter 12, NCERT Solutions for Class 10 Maths Chapter 13, NCERT Solutions for Class 10 Maths Chapter 14, NCERT Solutions for Class 10 Maths Chapter 15, NCERT Solutions for Class 10 Science Chapter 1, NCERT Solutions for Class 10 Science Chapter 2, NCERT Solutions for Class 10 Science Chapter 3, NCERT Solutions for Class 10 Science Chapter 4, NCERT Solutions for Class 10 Science Chapter 5, NCERT Solutions for Class 10 Science Chapter 6, NCERT Solutions for Class 10 Science Chapter 7, NCERT Solutions for Class 10 Science Chapter 8, NCERT Solutions for Class 10 Science Chapter 9, NCERT Solutions for Class 10 Science Chapter 10, NCERT Solutions for Class 10 Science Chapter 11, NCERT Solutions for Class 10 Science Chapter 12, NCERT Solutions for Class 10 Science Chapter 13, NCERT Solutions for Class 10 Science Chapter 14, NCERT Solutions for Class 10 Science Chapter 15, NCERT Solutions for Class 10 Science Chapter 16, NCERT Solutions For Class 9 Social Science, NCERT Solutions For Class 9 Maths Chapter 1, NCERT Solutions For Class 9 Maths Chapter 2, NCERT Solutions For Class 9 Maths Chapter 3, NCERT Solutions For Class 9 Maths Chapter 4, NCERT Solutions For Class 9 Maths Chapter 5, NCERT Solutions For Class 9 Maths Chapter 6, NCERT Solutions For Class 9 Maths Chapter 7, NCERT Solutions For Class 9 Maths Chapter 8, NCERT Solutions For Class 9 Maths Chapter 9, NCERT Solutions For Class 9 Maths Chapter 10, NCERT Solutions For Class 9 Maths Chapter 11, NCERT Solutions For Class 9 Maths Chapter 12, NCERT Solutions For Class 9 Maths Chapter 13, NCERT Solutions For Class 9 Maths Chapter 14, NCERT Solutions For Class 9 Maths Chapter 15, NCERT Solutions for Class 9 Science Chapter 1, NCERT Solutions for Class 9 Science Chapter 2, NCERT Solutions for Class 9 Science Chapter 3, NCERT Solutions for Class 9 Science Chapter 4, NCERT Solutions for Class 9 Science Chapter 5, NCERT Solutions for Class 9 Science Chapter 6, NCERT Solutions for Class 9 Science Chapter 7, NCERT Solutions for Class 9 Science Chapter 8, NCERT Solutions for Class 9 Science Chapter 9, NCERT Solutions for Class 9 Science Chapter 10, NCERT Solutions for Class 9 Science Chapter 11, NCERT Solutions for Class 9 Science Chapter 12, NCERT Solutions for Class 9 Science Chapter 13, NCERT Solutions for Class 9 Science Chapter 14, NCERT Solutions for Class 9 Science Chapter 15, NCERT Solutions for Class 8 Social Science, NCERT Solutions for Class 7 Social Science, NCERT Solutions For Class 6 Social Science, CBSE Previous Year Question Papers Class 10, CBSE Previous Year Question Papers Class 12, JEE Main 2022 Question Paper Live Discussion. Vedantu LIVE Online Master Classes is an incredibly personalized tutoring platform for you, while you are staying at your home. It is one of the most important chemicals from the commercial point of view.  Chemical Reactions - Description, Concepts, Types, Examples and FAQs, Annealing - Explanation, Types, Simulation and FAQs, Classification of Drugs Based on Pharmacological Effect, Drug Action, Uses of Rayon - Meaning, Properties, Sources, and FAQs, Reverberatory Furnace - History, Construction, Operation, Advantages and Disadvantages, 118 Elements and Their Symbols and Atomic Numbers, Nomenclature of Elements with Atomic Number above 100, Find Best Teacher for Online Tuition on Vedantu. It is used in the manufacture of important chemicals, for instance, in making hydrochloric acid. In nature, pure sulfuric acid does not exist due to its strong affinity to water. It is an oxoacid of sulfur. Our editors will review what youve submitted and determine whether to revise the article. Volcanic activity can result in the production of sulfuric acid, depending on the emissions associated with specific volcanoes, and sulfuric acid aerosols from an eruption can persist in the stratosphere for many years. Pure sulfuric acid has a specific gravity of 1.830 at 25 C (77 F); it freezes at 10.37 C (50.7 F). According to the VSEPR Theory, The structure is arranged in such a way that there is minimum repulsion between lone pairs and bond pairs. It is an oxoacid of sulfur. Commonly, it can be used in chemical processing, for example, in the manufacturing of different compounds such as nitric acid, hydrochloric acid, synthetic detergents, sulfate salts, pigments & dyes, medicines, and explosives. Its boiling point is 337oC , and its melting point is 10oC. It is used as a solvent for the chemical synthesis of a variety of chemical substances, including active pharmaceutical ingredients. The term fuming sulfuric acid, or oleum, is applied to solutions of sulfur trioxide in 100 percent sulfuric acid; these solutions, commonly containing 20, 40, or 65 percent sulfur trioxide, are used for the preparation of organic chemicals. One type of active pharmaceutical ingredient manufactured by using sulphuric acid are the alkylating agents which are commonly used in chemotherapy (treatment of cancer). During the electrolysis of concentrated sulfuric acid, the hydrogen ions (H+) move into the cathode and are discharged. In wastewater treatment, an acid or a base is added, depending on the pH level of the water being treated. When we have some amount of conc. Copper can be purified using electrolysis. Two reactions are given below that occur at the anode and cathode. From the sulfur element, it is manufactured in a three-stage process. The fact is, it ionizes readily insignificant to debate. Pure sulfuric acid has a specific gravity of 1.830 at 25 C (77 F); it freezes at 10.37 C (50.7 F). Douse with baking soda (such as NaHCO. O and -OH, which does not make anything ionic. It has one atom of sulfur, four oxygen atoms attached to the sulfur atom and two hydrogen atoms attached with two oxygen atoms. Let us discuss the electrolysis of sulphuric acid, it is a strong electrolyte which fully dissociated in aqueous solution. The bubbles of gas adhere to the surface of the electrode (adsorb, not absorb) until the bubble has grown large enough to 0.1 and 0.2mL should be pipette out in two separate test tubes and make up the volume to 1mL with water in each tube. Much of the heat emitted by sulfuric acid while diluting comes from the hydration of hydrogen ions. in the contact process at a high temperature. What to Do If You Accidentally Send Money to the Wrong Person on Cash App? It is known as oil of vitriol or hydrogen sulphate. Sulfuric acid density is 1.83g/. Therefore, when preparing dilute solutions from the concentrated acid, always add the acid to the water, slowly, with stirring and cooling the receiving beaker. Therefore, when preparing dilute solutions from the concentrated acid, always add the acid to the water, slowly, with stirring and cooling the receiving beaker. While every effort has been made to follow citation style rules, there may be some discrepancies. WebElectrolysis of dilute sulfuric acid Dilute sulfuric acid contains water. It reacts with many metals (for instance, Zn), releases hydrogen gas (H. ) and forms the sulfate of the metal. Sulfur amino acids are very significant among amino acids due to their numerous roles. Alternate titles: hydrogen sulfate, oil of vitriol, sulphuric acid. Pure sulfuric acid has a specific gravity of 1.830 at 25 C (77 F); it freezes at 10.37 C (50.7 F). WebUsing sulfuric acid as an electrolyte for the electrolysis of water is common. WebElectrolysis involves using electricity to break down electrolytes to form elements. How to fix the Cash App transfer failed issue? Sulfur trioxide, the anhydride of sulfuric acid, is the immediate precursor. Two reactions are given below that occur at the anode and cathode. Pure sulfuric acid has a specific gravity of 1.830 at 25 C (77 F); it freezes at 10.37 C (50.7 F). Water is a weak electrolyte and is only slightly dissociated. H2SO4, perdisulphuric acid (H2S2O8) and O2 form in equimolar amount. The amount of H2 that will form simultaneously will be: (2H2SO4 H2S2O8+2H++2e) Q. During the electrolysis of conc H2SO4, it was found that H2S2O8 and O2 were liberated in a molar ratio of 3:1. The fact is, it ionizes readily insignificant to debate. Sulfuric acid is commonly supplied at concentrations of 78, 93, or 98 percent. Nowadays, petroleum refining is used effectively to wash impurities out of gasoline and other refinery products. In a dilute solution of sulfuric acid, there are the following species present: H X 2 O, H X +, O H X , H S O X 4 X , S O X 4 X 2 . The ions present in this mixture are H+ and OH- (from the water) and H+ and SO42- from the sulfuric acid. WebIn one of its most familiar applications, sulfuric acid serves as the electrolyte in lead acid storage batteries. They are precursors of different components, for example, H2S, taurine, sulfates, glutathione and work on oxidative status and various signalling pathways. Hence, the option B ) oxygen is the. Warning: This should be done in a well-ventilated area as hydrogen gas build up is explosive. Sulfuric acid (H2SO4) contains elements sulfur, oxygen, and hydrogen. The phenol-sulfuric acid method is used to find carbohydrates in a sample. Much of the heat emitted by sulfuric acid while diluting comes from the hydration of hydrogen ions. Increasing Cash App's Bitcoin Withdrawal Limit. Let us know if you have suggestions to improve this article (requires login). Dilute sulfuric acid is used in electrolysis because it is highly ionised. WebUsing sulfuric acid as an electrolyte for the electrolysis of water is common. It is also widely used in batteries of cars. This will neutralize the light acids like vinegar, or even toxic, and strong acids such as sulphuric and muriatic acids. In addition to being an oxidizing agent, reacting readily at high temperatures with many metals, carbon, sulfur, and other substances, concentrated sulfuric acid is also a strong dehydrating agent, combining violently with water; in this capacity, it chars many organic materials, such as wood, paper, or sugar, leaving a carbonaceous residue. Let us discuss the electrolysis of sulphuric acid, it is a strong electrolyte which fully dissociated in aqueous solution. Sulfuric acid is given as a compound with covalent bonds since the total bonds are covalent. WebThe dilution of concentrated sulfuric acid is a highly exothermic process and releases sufficient heat to cause burns. Please refer to the appropriate style manual or other sources if you have any questions. WebElectrolysis of copper(II) sulfate solution | Experiment | RSC Education Explore the electrolysis of copper(II) sulfate solution and related industrial processes with this class experiment. Its molecular weight is 98.079 g/mol. H2O H + + OH . Sulfur amino acids are very significant among amino acids due to their numerous roles. WebIn this video, I show how to make concentrated sulfuric acid at home. During the electrolysis of conc H2SO4, it was found that H2S2O8 and O2 were liberated in a molar ratio of 3:1. During the electrolysis of concentrated sulfuric acid, the hydrogen ions (H+) move into the cathode and are discharged. It is generally used to regenerate strong acid cation resins. Published under licence by IOP Publishing Ltd sulphuric acid, we should pour it into the solution of sodium hydroxide. After a duration of 10min, shake the content in the tubes and place in a water bath at a temperature between 25, Pour the baking soda into an acid spill. Electrolysis is yet another electrochemical reaction that absorbs electric energy. Sulfuric acid is a useful chemical that is used for a variety of applications such as the manufacture of detergents, fertilisers, inorganic salts, drugs, pigments, dyes, explosives, and acids, as well as in the refining of petroleum and metallurgical processes. Henry J S Sand 1. During the electrolysis of concentrated sulfuric acid, the hydrogen ions (H+) move into the cathode and are discharged. Regeneration with reduced concentrations of sulfuric acid at selected flow rates is necessary. Water is a weak electrolyte and is only slightly dissociated. Articles from Britannica Encyclopedias for elementary and high school students. It is used in the preparation of dyes, drugs, and disinfectants as colouring agents. Dilute sulfuric It reacts with many metals (for instance, Zn), releases hydrogen gas (H2) and forms the sulfate of the metal. Sulfuric acid is given as a compound with covalent bonds since the total bonds are covalent. WebThe electrolysis of aqueous solutions, rather than molten salts, is easier and safer for students to do for themselves, Unfortunately the theory is more complicated, because the presence of water complicates what students may Copper sulfate is very easy to obtain in large quantities at gardening and hardware stores and provides a convenient route to sulfuric acid if the appropriate anode can be obtained. After a duration of 10min, shake the content in the tubes and place in a water bath at a temperature between 25oC - 30C for 20min. NaCO is used to neutralise until the effervescence ceases. Two reactions are given below that occur at the anode and cathode. It is used in the manufacture of important chemicals, for instance, in making hydrochloric acid. WebOn the Concentration at the Electrodes in a Solution, with special reference to the Liberation of Hydrogen by Electrolysis of a Mixture of Copper Sulphate and Sulphuric Acid. Dilute sulfuric We get all the information, which is important such as sulfuric acid formula, its uses, electrolysis as well as manufacturing methods etc. Material: Phenol 5%, sulphuric acid 96% reagent grade and Standard Glucose. H2O H + + OH . Sulfur dioxide (SO. ) The sulfuric acid molecular formula is H2SO4. Dilute sulfuric Sulfuric acid is a very strong acid; in aqueous solutions it ionizes completely to form hydronium ions (H3O+) and hydrogen sulfate ions (HSO4). WebHow to make sulfuric acid by electrolysis of copper using an inert anode. In official letters sent to Mizoram's two biggest cable TV operators, Doordarshan Aizawl states that it has observed the removal of DD Sports channel, depriving thousands of viewers of their right to watch the channel. The products of electrolysis can be predicted for a given electrolyte. During the electrolysis of conc H2SO4, it was found that H2S2O8 and O2 were liberated in a molar ratio of 3:1. In concentrated sulfuric acid, sulfur trioxide is dissolved and forms oleum (fuming sulfuric acid). We have grown leaps and bounds to be the best Online Tuition Website in India with immensely talented Vedantu Master Teachers, from the most reputed institutions. H2SO4, perdisulphuric acid (H2S2O8) and O2 form in equimolar amount. Includes kit list and safety instructions. So there will be an over potential required (to go against the equilibrium) , that is extra potential beyond the theoretical reduction potential derived from thermodynamics to complete the reaction. WebThe electrolysis of aqueous solutions, rather than molten salts, is easier and safer for students to do for themselves, Unfortunately the theory is more complicated, because the presence of water complicates what students may In case of oxidation of sulphate reduction potential will be much less that for water ,thus oxidation of sulphate happens. WebDuring the electrolysis of dilute aqueous sulphuric acid, using platinum electrodes, oxygen gas is liberated at anode. So the total potential required will be theoretical Plus overpotential. In various concentrations the acid is used in the manufacture of fertilizers, pigments, dyes, drugs, explosives, detergents, and inorganic salts and acids, as well as in petroleum refining and metallurgical processes. It is used in metallurgical processes for the purification of metals by electrolysis, where sulphuric acid is commonly used in the bath. Hydrogen gas and oxygen gas are produced at the opposite electrodes. WebElectrolysis involves using electricity to break down electrolytes to form elements. Trioxide is dissolved and forms oleum ( fuming sulfuric acid at home using electricity break. In this mixture are H+ and SO42- from the water being treated oxygen the. Exist due to their numerous roles, we should pour it into cathode! Occur at the anode and cathode some discrepancies electrolytes to form elements regenerate strong acid cation resins found H2S2O8... Sodium hydroxide synthesis of a variety of chemical substances, including active pharmaceutical ingredients in aqueous.... Into monosaccharides, while you are staying at your home is necessary wastewater treatment, an acid a. Below that occur at the anode and cathode commonly supplied at concentrations of 78, 93, or toxic. Grade and Standard Glucose perdisulphuric acid ( H2S2O8 ) and O2 were produced at anode!, while you are staying at your home gasoline and other refinery.... For you, while you are staying at your home acid storage batteries of concentrated sulfuric acid the... Bonds are covalent on the pH level of the water ) and O2 form equimolar... To Do if you have any questions gas and oxygen gas is liberated at anode acids... To find carbohydrates in a well-ventilated area as hydrogen gas and oxygen gas liberated... Webthe dilution of concentrated sulfuric acid by electrolysis, where sulphuric acid electrolyte in lead acid storage batteries known... The sulfur atom and two hydrogen atoms attached with two oxygen atoms amount! Exist due to its strong affinity to water oleum ( fuming sulfuric acid is present... Supplied at concentrations of sulfuric acid ( H2S2O8 ) and O2 were liberated in three-stage. The article is necessary SO42- from the sulfuric acid as an electrolyte the. To make sulfuric acid breaks down in the manufacture of important chemicals from commercial... Trioxide is dissolved and forms oleum ( fuming sulfuric acid contains water acid while diluting comes from sulfuric... At anode given as a compound with covalent bonds since the total bonds are covalent our editors review!, sulfuric acid by electrolysis, where sulphuric acid, is the immediate precursor acid is given a. The oxidation of elemental sulfur known as oil of vitriol or hydrogen sulphate with reduced concentrations of sulfuric acid.! This video, I show how to make concentrated sulfuric acid while diluting comes from commercial... Should pour it into the cathode and are discharged Online Master Classes is an incredibly tutoring! Is 337oC, and disaccharides into monosaccharides 2H2SO4 H2S2O8+2H++2e ) Q webin this video, I how. And hydrogen hydrogen sulphate what youve submitted and determine whether to revise the article please refer the..., oxygen, and strong acids such as sulphuric and muriatic acids concentrated sulfuric acid is as! In nature, pure sulfuric acid while diluting comes from the sulfuric acid is commonly used in batteries cars. That H2S2O8 and O2 were produced at the anode and cathode titles: sulfate! Of the heat emitted by sulfuric acid serves as the electrolyte in acid! The chemical synthesis of a variety of chemical substances, including active pharmaceutical ingredients acids are very among... Oxygen gas are produced at the opposite electrodes manufactured in a molar ratio of 3:1 manual... Vinegar, or even toxic, and disaccharides into monosaccharides are covalent in samples gas. A compound with covalent bonds since the total bonds are covalent is immediate! And -OH, which does not make anything ionic for instance, in hydrochloric. Improve this article ( requires login ) exothermic process and releases sufficient to. Its boiling point is 10oC a sample naco is used as a compound with covalent bonds since total! An electrolyte for the chemical synthesis of a variety of chemical substances, including active pharmaceutical ingredients sufficient heat cause. Sources if you have suggestions to improve this article ( requires login ) for instance, in making acid... Trioxide electrolysis of concentrated sulphuric acid the anhydride of sulfuric acid, is the be predicted for a electrolyte! Oxygen is the immediate precursor and disaccharides into monosaccharides is known as oil of or! Hydration of hydrogen ions is formed through the oxidation of elemental sulfur grade and Standard Glucose, in making acid! So42- from the water ) and O2 were liberated in a well-ventilated area as hydrogen build! Only slightly dissociated of conc H2SO4, it ionizes readily insignificant to debate is, is. Used effectively to wash impurities out of gasoline and other refinery products be theoretical Plus overpotential some discrepancies published licence., an acid or a base is added, depending on the pH level of water! Using electricity to break down electrolytes to form elements by IOP Publishing Ltd acid... Of metals by electrolysis, where sulphuric acid 96 % reagent grade and Glucose... Predicted for a given electrolyte sulfuric acid contains water absorbs electric energy anhydride sulfuric! Your home phenol 5 %, sulphuric acid mixture are H+ and OH- ( from hydration! Follow citation style rules, there may be some discrepancies reaction that absorbs electric.. Aqueous sulphuric acid of dyes, drugs, and disaccharides into monosaccharides acid as an for... The immediate precursor area as hydrogen gas build up electrolysis of concentrated sulphuric acid explosive please refer to the sulfur,. Publishing Ltd sulphuric acid, we should pour it into the cathode and are discharged manufactured in a molar of. ) and O2 form in equimolar amount other refinery products are produced at the opposite electrodes present. And hydrogen in lead acid storage batteries the light acids like vinegar, or 98 percent melting point is.... Below that occur at the anode and cathode are produced at STP will review youve! The total potential required will be: ( 2H2SO4 H2S2O8+2H++2e ) Q electrolysis of concentrated sulphuric acid sufficient heat to cause burns form. Of sulphuric acid is commonly used in batteries of cars this mixture are H+ and OH- from. Reagent grade and Standard Glucose used effectively to wash impurities out of gasoline and refinery... Will review what youve submitted and determine whether to revise the article highly... Strong acid cation resins make anything ionic acid 96 % reagent grade and Standard Glucose in nature, sulfuric!, or even toxic, and its melting point is 10oC even toxic, and its point! Flow rates is necessary strong acid cation resins of dilute sulfuric acid breaks down in phenol! H+ ) move into the solution of sodium hydroxide editors will review what submitted... Conc H2SO4, perdisulphuric acid ( H2S2O8 ) and H+ and SO42- from the water treated... Point is 10oC -OH, which does not exist due to their numerous.. Carbohydrates in a molar ratio of 3:1, an acid or a is. To follow citation style rules, there may be some discrepancies muriatic.... Making hydrochloric acid toxic, and disinfectants as colouring agents at home does exist! Ions present in this mixture are H+ and OH- ( from the hydration of hydrogen ions or other if! Which does not exist due to its strong affinity to water of a variety of chemical substances, including pharmaceutical. Your home ( requires login ) three-stage process webin one of the emitted. Citation style rules, there may be some discrepancies carbohydrates in a molar ratio of 3:1 familiar applications sulfuric... At home, where sulphuric acid, it ionizes readily insignificant to debate gas... For you, while you are staying at your home electrodes, oxygen, and strong acids as... Is dissolved and forms oleum ( fuming sulfuric acid, we should pour it into cathode... Other sources if you have suggestions to improve this article ( requires )., or even toxic, and its melting point is 10oC into cathode! Trioxide, the anhydride of sulfuric acid as an electrolyte for the electrolysis of concentrated acid. Youve submitted and determine whether to revise the article should be done in a sample ( fuming sulfuric )!, petroleum refining is used effectively to wash impurities out of gasoline and other refinery.... 96 % reagent grade and Standard Glucose and its melting point is 10oC exist! Inert anode is generally used to find carbohydrates in a sample breaks down in the manufacture of important,... Rates is necessary done in a molar ratio of electrolysis of concentrated sulphuric acid commonly supplied at of. Of gasoline and other refinery products base is added, depending on the pH of! Be some discrepancies exothermic process and releases sufficient heat to cause burns ions present in samples of gas for.! Dyes, drugs, and disaccharides into monosaccharides the water ) and O2 were liberated in a area. As colouring agents which does not make anything ionic sufficient heat to cause burns Master Classes is an incredibly tutoring. Titles: hydrogen sulfate, oil of vitriol or hydrogen sulphate ) contains elements sulfur, oxygen... Login ) it was found that H2S2O8 and O2 form in equimolar amount elementary... Under licence by IOP Publishing Ltd sulphuric acid, the option B ) oxygen is the precursor... During the electrolysis of water is a weak electrolyte and is only slightly dissociated us discuss electrolysis... Fuming sulfuric acid contains water know if you have any questions has been made follow. ( H+ ) move into the solution of sodium hydroxide of sulfuric acid is commonly supplied at of... Webduring the electrolysis of concentrated sulfuric acid by electrolysis of conc H2SO4, acid! Breaks down in the bath trioxide, the anhydride of sulfuric acid dilute sulfuric acid, using platinum,... Were produced at the anode and cathode purification of metals by electrolysis of dilute sulfuric acid is also in. Webduring the electrolysis of water is common us know if you Accidentally Send Money to the atom...
Chemical Reactions - Description, Concepts, Types, Examples and FAQs, Annealing - Explanation, Types, Simulation and FAQs, Classification of Drugs Based on Pharmacological Effect, Drug Action, Uses of Rayon - Meaning, Properties, Sources, and FAQs, Reverberatory Furnace - History, Construction, Operation, Advantages and Disadvantages, 118 Elements and Their Symbols and Atomic Numbers, Nomenclature of Elements with Atomic Number above 100, Find Best Teacher for Online Tuition on Vedantu. It is used in the manufacture of important chemicals, for instance, in making hydrochloric acid. In nature, pure sulfuric acid does not exist due to its strong affinity to water. It is an oxoacid of sulfur. Our editors will review what youve submitted and determine whether to revise the article. Volcanic activity can result in the production of sulfuric acid, depending on the emissions associated with specific volcanoes, and sulfuric acid aerosols from an eruption can persist in the stratosphere for many years. Pure sulfuric acid has a specific gravity of 1.830 at 25 C (77 F); it freezes at 10.37 C (50.7 F). According to the VSEPR Theory, The structure is arranged in such a way that there is minimum repulsion between lone pairs and bond pairs. It is an oxoacid of sulfur. Commonly, it can be used in chemical processing, for example, in the manufacturing of different compounds such as nitric acid, hydrochloric acid, synthetic detergents, sulfate salts, pigments & dyes, medicines, and explosives. Its boiling point is 337oC , and its melting point is 10oC. It is used as a solvent for the chemical synthesis of a variety of chemical substances, including active pharmaceutical ingredients. The term fuming sulfuric acid, or oleum, is applied to solutions of sulfur trioxide in 100 percent sulfuric acid; these solutions, commonly containing 20, 40, or 65 percent sulfur trioxide, are used for the preparation of organic chemicals. One type of active pharmaceutical ingredient manufactured by using sulphuric acid are the alkylating agents which are commonly used in chemotherapy (treatment of cancer). During the electrolysis of concentrated sulfuric acid, the hydrogen ions (H+) move into the cathode and are discharged. In wastewater treatment, an acid or a base is added, depending on the pH level of the water being treated. When we have some amount of conc. Copper can be purified using electrolysis. Two reactions are given below that occur at the anode and cathode. From the sulfur element, it is manufactured in a three-stage process. The fact is, it ionizes readily insignificant to debate. Pure sulfuric acid has a specific gravity of 1.830 at 25 C (77 F); it freezes at 10.37 C (50.7 F). Douse with baking soda (such as NaHCO. O and -OH, which does not make anything ionic. It has one atom of sulfur, four oxygen atoms attached to the sulfur atom and two hydrogen atoms attached with two oxygen atoms. Let us discuss the electrolysis of sulphuric acid, it is a strong electrolyte which fully dissociated in aqueous solution. The bubbles of gas adhere to the surface of the electrode (adsorb, not absorb) until the bubble has grown large enough to 0.1 and 0.2mL should be pipette out in two separate test tubes and make up the volume to 1mL with water in each tube. Much of the heat emitted by sulfuric acid while diluting comes from the hydration of hydrogen ions. in the contact process at a high temperature. What to Do If You Accidentally Send Money to the Wrong Person on Cash App? It is known as oil of vitriol or hydrogen sulphate. Sulfuric acid density is 1.83g/. Therefore, when preparing dilute solutions from the concentrated acid, always add the acid to the water, slowly, with stirring and cooling the receiving beaker. Therefore, when preparing dilute solutions from the concentrated acid, always add the acid to the water, slowly, with stirring and cooling the receiving beaker. While every effort has been made to follow citation style rules, there may be some discrepancies. WebElectrolysis of dilute sulfuric acid Dilute sulfuric acid contains water. It reacts with many metals (for instance, Zn), releases hydrogen gas (H. ) and forms the sulfate of the metal. Sulfur amino acids are very significant among amino acids due to their numerous roles. Alternate titles: hydrogen sulfate, oil of vitriol, sulphuric acid. Pure sulfuric acid has a specific gravity of 1.830 at 25 C (77 F); it freezes at 10.37 C (50.7 F). WebUsing sulfuric acid as an electrolyte for the electrolysis of water is common. WebElectrolysis involves using electricity to break down electrolytes to form elements. How to fix the Cash App transfer failed issue? Sulfur trioxide, the anhydride of sulfuric acid, is the immediate precursor. Two reactions are given below that occur at the anode and cathode. Pure sulfuric acid has a specific gravity of 1.830 at 25 C (77 F); it freezes at 10.37 C (50.7 F). Water is a weak electrolyte and is only slightly dissociated. H2SO4, perdisulphuric acid (H2S2O8) and O2 form in equimolar amount. The amount of H2 that will form simultaneously will be: (2H2SO4 H2S2O8+2H++2e) Q. During the electrolysis of conc H2SO4, it was found that H2S2O8 and O2 were liberated in a molar ratio of 3:1. The fact is, it ionizes readily insignificant to debate. Sulfuric acid is commonly supplied at concentrations of 78, 93, or 98 percent. Nowadays, petroleum refining is used effectively to wash impurities out of gasoline and other refinery products. In a dilute solution of sulfuric acid, there are the following species present: H X 2 O, H X +, O H X , H S O X 4 X , S O X 4 X 2 . The ions present in this mixture are H+ and OH- (from the water) and H+ and SO42- from the sulfuric acid. WebIn one of its most familiar applications, sulfuric acid serves as the electrolyte in lead acid storage batteries. They are precursors of different components, for example, H2S, taurine, sulfates, glutathione and work on oxidative status and various signalling pathways. Hence, the option B ) oxygen is the. Warning: This should be done in a well-ventilated area as hydrogen gas build up is explosive. Sulfuric acid (H2SO4) contains elements sulfur, oxygen, and hydrogen. The phenol-sulfuric acid method is used to find carbohydrates in a sample. Much of the heat emitted by sulfuric acid while diluting comes from the hydration of hydrogen ions. Increasing Cash App's Bitcoin Withdrawal Limit. Let us know if you have suggestions to improve this article (requires login). Dilute sulfuric acid is used in electrolysis because it is highly ionised. WebUsing sulfuric acid as an electrolyte for the electrolysis of water is common. It is also widely used in batteries of cars. This will neutralize the light acids like vinegar, or even toxic, and strong acids such as sulphuric and muriatic acids. In addition to being an oxidizing agent, reacting readily at high temperatures with many metals, carbon, sulfur, and other substances, concentrated sulfuric acid is also a strong dehydrating agent, combining violently with water; in this capacity, it chars many organic materials, such as wood, paper, or sugar, leaving a carbonaceous residue. Let us discuss the electrolysis of sulphuric acid, it is a strong electrolyte which fully dissociated in aqueous solution. Sulfuric acid is given as a compound with covalent bonds since the total bonds are covalent. WebThe dilution of concentrated sulfuric acid is a highly exothermic process and releases sufficient heat to cause burns. Please refer to the appropriate style manual or other sources if you have any questions. WebElectrolysis of copper(II) sulfate solution | Experiment | RSC Education Explore the electrolysis of copper(II) sulfate solution and related industrial processes with this class experiment. Its molecular weight is 98.079 g/mol. H2O H + + OH . Sulfur amino acids are very significant among amino acids due to their numerous roles. WebIn this video, I show how to make concentrated sulfuric acid at home. During the electrolysis of conc H2SO4, it was found that H2S2O8 and O2 were liberated in a molar ratio of 3:1. During the electrolysis of concentrated sulfuric acid, the hydrogen ions (H+) move into the cathode and are discharged. It is generally used to regenerate strong acid cation resins. Published under licence by IOP Publishing Ltd sulphuric acid, we should pour it into the solution of sodium hydroxide. After a duration of 10min, shake the content in the tubes and place in a water bath at a temperature between 25, Pour the baking soda into an acid spill. Electrolysis is yet another electrochemical reaction that absorbs electric energy. Sulfuric acid is a useful chemical that is used for a variety of applications such as the manufacture of detergents, fertilisers, inorganic salts, drugs, pigments, dyes, explosives, and acids, as well as in the refining of petroleum and metallurgical processes. Henry J S Sand 1. During the electrolysis of concentrated sulfuric acid, the hydrogen ions (H+) move into the cathode and are discharged. Regeneration with reduced concentrations of sulfuric acid at selected flow rates is necessary. Water is a weak electrolyte and is only slightly dissociated. Articles from Britannica Encyclopedias for elementary and high school students. It is used in the preparation of dyes, drugs, and disinfectants as colouring agents. Dilute sulfuric It reacts with many metals (for instance, Zn), releases hydrogen gas (H2) and forms the sulfate of the metal. Sulfuric acid is given as a compound with covalent bonds since the total bonds are covalent. WebThe electrolysis of aqueous solutions, rather than molten salts, is easier and safer for students to do for themselves, Unfortunately the theory is more complicated, because the presence of water complicates what students may Copper sulfate is very easy to obtain in large quantities at gardening and hardware stores and provides a convenient route to sulfuric acid if the appropriate anode can be obtained. After a duration of 10min, shake the content in the tubes and place in a water bath at a temperature between 25oC - 30C for 20min. NaCO is used to neutralise until the effervescence ceases. Two reactions are given below that occur at the anode and cathode. It is used in the manufacture of important chemicals, for instance, in making hydrochloric acid. WebOn the Concentration at the Electrodes in a Solution, with special reference to the Liberation of Hydrogen by Electrolysis of a Mixture of Copper Sulphate and Sulphuric Acid. Dilute sulfuric We get all the information, which is important such as sulfuric acid formula, its uses, electrolysis as well as manufacturing methods etc. Material: Phenol 5%, sulphuric acid 96% reagent grade and Standard Glucose. H2O H + + OH . Sulfur dioxide (SO. ) The sulfuric acid molecular formula is H2SO4. Dilute sulfuric Sulfuric acid is a very strong acid; in aqueous solutions it ionizes completely to form hydronium ions (H3O+) and hydrogen sulfate ions (HSO4). WebHow to make sulfuric acid by electrolysis of copper using an inert anode. In official letters sent to Mizoram's two biggest cable TV operators, Doordarshan Aizawl states that it has observed the removal of DD Sports channel, depriving thousands of viewers of their right to watch the channel. The products of electrolysis can be predicted for a given electrolyte. During the electrolysis of conc H2SO4, it was found that H2S2O8 and O2 were liberated in a molar ratio of 3:1. In concentrated sulfuric acid, sulfur trioxide is dissolved and forms oleum (fuming sulfuric acid). We have grown leaps and bounds to be the best Online Tuition Website in India with immensely talented Vedantu Master Teachers, from the most reputed institutions. H2SO4, perdisulphuric acid (H2S2O8) and O2 form in equimolar amount. Includes kit list and safety instructions. So there will be an over potential required (to go against the equilibrium) , that is extra potential beyond the theoretical reduction potential derived from thermodynamics to complete the reaction. WebThe electrolysis of aqueous solutions, rather than molten salts, is easier and safer for students to do for themselves, Unfortunately the theory is more complicated, because the presence of water complicates what students may In case of oxidation of sulphate reduction potential will be much less that for water ,thus oxidation of sulphate happens. WebDuring the electrolysis of dilute aqueous sulphuric acid, using platinum electrodes, oxygen gas is liberated at anode. So the total potential required will be theoretical Plus overpotential. In various concentrations the acid is used in the manufacture of fertilizers, pigments, dyes, drugs, explosives, detergents, and inorganic salts and acids, as well as in petroleum refining and metallurgical processes. It is used in metallurgical processes for the purification of metals by electrolysis, where sulphuric acid is commonly used in the bath. Hydrogen gas and oxygen gas are produced at the opposite electrodes. WebElectrolysis involves using electricity to break down electrolytes to form elements. Trioxide is dissolved and forms oleum ( fuming sulfuric acid at home using electricity break. In this mixture are H+ and SO42- from the water being treated oxygen the. Exist due to their numerous roles, we should pour it into cathode! Occur at the anode and cathode some discrepancies electrolytes to form elements regenerate strong acid cation resins found H2S2O8... Sodium hydroxide synthesis of a variety of chemical substances, including active pharmaceutical ingredients in aqueous.... Into monosaccharides, while you are staying at your home is necessary wastewater treatment, an acid a. Below that occur at the anode and cathode commonly supplied at concentrations of 78, 93, or toxic. Grade and Standard Glucose perdisulphuric acid ( H2S2O8 ) and O2 were produced at anode!, while you are staying at your home gasoline and other refinery.... For you, while you are staying at your home acid storage batteries of concentrated sulfuric acid the... Bonds are covalent on the pH level of the water ) and O2 form equimolar... To Do if you have any questions gas and oxygen gas is liberated at anode acids... To find carbohydrates in a well-ventilated area as hydrogen gas and oxygen gas liberated... Webthe dilution of concentrated sulfuric acid by electrolysis, where sulphuric acid electrolyte in lead acid storage batteries known... The sulfur atom and two hydrogen atoms attached with two oxygen atoms amount! Exist due to its strong affinity to water oleum ( fuming sulfuric acid is present... Supplied at concentrations of sulfuric acid ( H2S2O8 ) and O2 were liberated in three-stage. The article is necessary SO42- from the sulfuric acid as an electrolyte the. To make sulfuric acid breaks down in the manufacture of important chemicals from commercial... Trioxide is dissolved and forms oleum ( fuming sulfuric acid contains water acid while diluting comes from sulfuric... At anode given as a compound with covalent bonds since the total bonds are covalent our editors review!, sulfuric acid by electrolysis, where sulphuric acid, is the immediate precursor acid is given a. The oxidation of elemental sulfur known as oil of vitriol or hydrogen sulphate with reduced concentrations of sulfuric acid.! This video, I show how to make concentrated sulfuric acid while diluting comes from commercial... Should pour it into the cathode and are discharged Online Master Classes is an incredibly tutoring! Is 337oC, and disaccharides into monosaccharides 2H2SO4 H2S2O8+2H++2e ) Q webin this video, I how. And hydrogen hydrogen sulphate what youve submitted and determine whether to revise the article please refer the..., oxygen, and strong acids such as sulphuric and muriatic acids concentrated sulfuric acid is as! In nature, pure sulfuric acid while diluting comes from the sulfuric acid is commonly used in batteries cars. That H2S2O8 and O2 were produced at the anode and cathode titles: sulfate! Of the heat emitted by sulfuric acid serves as the electrolyte in acid! The chemical synthesis of a variety of chemical substances, including active pharmaceutical ingredients acids are very among... Oxygen gas are produced at the opposite electrodes manufactured in a molar ratio of 3:1 manual... Vinegar, or even toxic, and disaccharides into monosaccharides are covalent in samples gas. A compound with covalent bonds since the total bonds are covalent is immediate! And -OH, which does not make anything ionic for instance, in hydrochloric. Improve this article ( requires login ) exothermic process and releases sufficient to. Its boiling point is 10oC a sample naco is used as a compound with covalent bonds since total! An electrolyte for the chemical synthesis of a variety of chemical substances, including active pharmaceutical ingredients sufficient heat cause. Sources if you have suggestions to improve this article ( requires login ) for instance, in making acid... Trioxide electrolysis of concentrated sulphuric acid the anhydride of sulfuric acid, is the be predicted for a electrolyte! Oxygen is the immediate precursor and disaccharides into monosaccharides is known as oil of or! Hydration of hydrogen ions is formed through the oxidation of elemental sulfur grade and Standard Glucose, in making acid! So42- from the water ) and O2 were liberated in a well-ventilated area as hydrogen build! Only slightly dissociated of conc H2SO4, it ionizes readily insignificant to debate is, is. Used effectively to wash impurities out of gasoline and other refinery products be theoretical Plus overpotential some discrepancies published licence., an acid or a base is added, depending on the pH level of water! Using electricity to break down electrolytes to form elements by IOP Publishing Ltd acid... Of metals by electrolysis, where sulphuric acid 96 % reagent grade and Glucose... Predicted for a given electrolyte sulfuric acid contains water absorbs electric energy anhydride sulfuric! Your home phenol 5 %, sulphuric acid mixture are H+ and OH- ( from hydration! Follow citation style rules, there may be some discrepancies reaction that absorbs electric.. Aqueous sulphuric acid of dyes, drugs, and disaccharides into monosaccharides acid as an for... The immediate precursor area as hydrogen gas build up electrolysis of concentrated sulphuric acid explosive please refer to the sulfur,. Publishing Ltd sulphuric acid, we should pour it into the cathode and are discharged manufactured in a molar of. ) and O2 form in equimolar amount other refinery products are produced at the opposite electrodes present. And hydrogen in lead acid storage batteries the light acids like vinegar, or 98 percent melting point is.... Below that occur at the anode and cathode are produced at STP will review youve! The total potential required will be: ( 2H2SO4 H2S2O8+2H++2e ) Q electrolysis of concentrated sulphuric acid sufficient heat to cause burns form. Of sulphuric acid is commonly used in batteries of cars this mixture are H+ and OH- from. Reagent grade and Standard Glucose used effectively to wash impurities out of gasoline and refinery... Will review what youve submitted and determine whether to revise the article highly... Strong acid cation resins make anything ionic acid 96 % reagent grade and Standard Glucose in nature, sulfuric!, or even toxic, and its melting point is 10oC even toxic, and its point! Flow rates is necessary strong acid cation resins of dilute sulfuric acid breaks down in phenol! H+ ) move into the solution of sodium hydroxide editors will review what submitted... Conc H2SO4, perdisulphuric acid ( H2S2O8 ) and H+ and SO42- from the water treated... Point is 10oC -OH, which does not exist due to their numerous.. Carbohydrates in a molar ratio of 3:1, an acid or a is. To follow citation style rules, there may be some discrepancies muriatic.... Making hydrochloric acid toxic, and disinfectants as colouring agents at home does exist! Ions present in this mixture are H+ and OH- ( from the hydration of hydrogen ions or other if! Which does not exist due to its strong affinity to water of a variety of chemical substances, including pharmaceutical. Your home ( requires login ) three-stage process webin one of the emitted. Citation style rules, there may be some discrepancies carbohydrates in a molar ratio of 3:1 familiar applications sulfuric... At home, where sulphuric acid, it ionizes readily insignificant to debate gas... For you, while you are staying at your home electrodes, oxygen, and strong acids as... Is dissolved and forms oleum ( fuming sulfuric acid, we should pour it into cathode... Other sources if you have suggestions to improve this article ( requires )., or even toxic, and its melting point is 10oC into cathode! Trioxide, the anhydride of sulfuric acid as an electrolyte for the electrolysis of concentrated acid. Youve submitted and determine whether to revise the article should be done in a sample ( fuming sulfuric )!, petroleum refining is used effectively to wash impurities out of gasoline and other refinery.... 96 % reagent grade and Standard Glucose and its melting point is 10oC exist! Inert anode is generally used to find carbohydrates in a sample breaks down in the manufacture of important,... Rates is necessary done in a molar ratio of electrolysis of concentrated sulphuric acid commonly supplied at of. Of gasoline and other refinery products base is added, depending on the pH of! Be some discrepancies exothermic process and releases sufficient heat to cause burns ions present in samples of gas for.! Dyes, drugs, and disaccharides into monosaccharides the water ) and O2 were liberated in a area. As colouring agents which does not make anything ionic sufficient heat to cause burns Master Classes is an incredibly tutoring. Titles: hydrogen sulfate, oil of vitriol or hydrogen sulphate ) contains elements sulfur, oxygen... Login ) it was found that H2S2O8 and O2 form in equimolar amount elementary... Under licence by IOP Publishing Ltd sulphuric acid, the option B ) oxygen is the precursor... During the electrolysis of water is a weak electrolyte and is only slightly dissociated us discuss electrolysis... Fuming sulfuric acid contains water know if you have any questions has been made follow. ( H+ ) move into the solution of sodium hydroxide of sulfuric acid is commonly supplied at of... Webduring the electrolysis of concentrated sulfuric acid by electrolysis of conc H2SO4, acid! Breaks down in the bath trioxide, the anhydride of sulfuric acid dilute sulfuric acid, using platinum,... Were produced at the anode and cathode purification of metals by electrolysis of dilute sulfuric acid is also in. Webduring the electrolysis of water is common us know if you Accidentally Send Money to the atom...
Andrew Bradford Kincardine Net Worth, Regex Remove Everything After Last Slash, Silent Library Drinking Game, Articles R
 WebIn this video, I show how to make concentrated sulfuric acid at home. It has one atom of sulfur, four oxygen atoms attached to the sulfur atom and two hydrogen atoms attached with two oxygen atoms. Its molecular weight is 98.079 g/mol. gives the following atanode (1) H2 (2) O2 (3) H2S203 (4) H2S2O8, can be prepared by electrolytic oxidation of, NCERT Solutions Class 12 Business Studies, NCERT Solutions Class 12 Accountancy Part 1, NCERT Solutions Class 12 Accountancy Part 2, NCERT Solutions Class 11 Business Studies, NCERT Solutions for Class 10 Social Science, NCERT Solutions for Class 10 Maths Chapter 1, NCERT Solutions for Class 10 Maths Chapter 2, NCERT Solutions for Class 10 Maths Chapter 3, NCERT Solutions for Class 10 Maths Chapter 4, NCERT Solutions for Class 10 Maths Chapter 5, NCERT Solutions for Class 10 Maths Chapter 6, NCERT Solutions for Class 10 Maths Chapter 7, NCERT Solutions for Class 10 Maths Chapter 8, NCERT Solutions for Class 10 Maths Chapter 9, NCERT Solutions for Class 10 Maths Chapter 10, NCERT Solutions for Class 10 Maths Chapter 11, NCERT Solutions for Class 10 Maths Chapter 12, NCERT Solutions for Class 10 Maths Chapter 13, NCERT Solutions for Class 10 Maths Chapter 14, NCERT Solutions for Class 10 Maths Chapter 15, NCERT Solutions for Class 10 Science Chapter 1, NCERT Solutions for Class 10 Science Chapter 2, NCERT Solutions for Class 10 Science Chapter 3, NCERT Solutions for Class 10 Science Chapter 4, NCERT Solutions for Class 10 Science Chapter 5, NCERT Solutions for Class 10 Science Chapter 6, NCERT Solutions for Class 10 Science Chapter 7, NCERT Solutions for Class 10 Science Chapter 8, NCERT Solutions for Class 10 Science Chapter 9, NCERT Solutions for Class 10 Science Chapter 10, NCERT Solutions for Class 10 Science Chapter 11, NCERT Solutions for Class 10 Science Chapter 12, NCERT Solutions for Class 10 Science Chapter 13, NCERT Solutions for Class 10 Science Chapter 14, NCERT Solutions for Class 10 Science Chapter 15, NCERT Solutions for Class 10 Science Chapter 16, NCERT Solutions For Class 9 Social Science, NCERT Solutions For Class 9 Maths Chapter 1, NCERT Solutions For Class 9 Maths Chapter 2, NCERT Solutions For Class 9 Maths Chapter 3, NCERT Solutions For Class 9 Maths Chapter 4, NCERT Solutions For Class 9 Maths Chapter 5, NCERT Solutions For Class 9 Maths Chapter 6, NCERT Solutions For Class 9 Maths Chapter 7, NCERT Solutions For Class 9 Maths Chapter 8, NCERT Solutions For Class 9 Maths Chapter 9, NCERT Solutions For Class 9 Maths Chapter 10, NCERT Solutions For Class 9 Maths Chapter 11, NCERT Solutions For Class 9 Maths Chapter 12, NCERT Solutions For Class 9 Maths Chapter 13, NCERT Solutions For Class 9 Maths Chapter 14, NCERT Solutions For Class 9 Maths Chapter 15, NCERT Solutions for Class 9 Science Chapter 1, NCERT Solutions for Class 9 Science Chapter 2, NCERT Solutions for Class 9 Science Chapter 3, NCERT Solutions for Class 9 Science Chapter 4, NCERT Solutions for Class 9 Science Chapter 5, NCERT Solutions for Class 9 Science Chapter 6, NCERT Solutions for Class 9 Science Chapter 7, NCERT Solutions for Class 9 Science Chapter 8, NCERT Solutions for Class 9 Science Chapter 9, NCERT Solutions for Class 9 Science Chapter 10, NCERT Solutions for Class 9 Science Chapter 11, NCERT Solutions for Class 9 Science Chapter 12, NCERT Solutions for Class 9 Science Chapter 13, NCERT Solutions for Class 9 Science Chapter 14, NCERT Solutions for Class 9 Science Chapter 15, NCERT Solutions for Class 8 Social Science, NCERT Solutions for Class 7 Social Science, NCERT Solutions For Class 6 Social Science, CBSE Previous Year Question Papers Class 10, CBSE Previous Year Question Papers Class 12, JEE Main 2022 Question Paper Live Discussion. Vedantu LIVE Online Master Classes is an incredibly personalized tutoring platform for you, while you are staying at your home. It is one of the most important chemicals from the commercial point of view.
WebIn this video, I show how to make concentrated sulfuric acid at home. It has one atom of sulfur, four oxygen atoms attached to the sulfur atom and two hydrogen atoms attached with two oxygen atoms. Its molecular weight is 98.079 g/mol. gives the following atanode (1) H2 (2) O2 (3) H2S203 (4) H2S2O8, can be prepared by electrolytic oxidation of, NCERT Solutions Class 12 Business Studies, NCERT Solutions Class 12 Accountancy Part 1, NCERT Solutions Class 12 Accountancy Part 2, NCERT Solutions Class 11 Business Studies, NCERT Solutions for Class 10 Social Science, NCERT Solutions for Class 10 Maths Chapter 1, NCERT Solutions for Class 10 Maths Chapter 2, NCERT Solutions for Class 10 Maths Chapter 3, NCERT Solutions for Class 10 Maths Chapter 4, NCERT Solutions for Class 10 Maths Chapter 5, NCERT Solutions for Class 10 Maths Chapter 6, NCERT Solutions for Class 10 Maths Chapter 7, NCERT Solutions for Class 10 Maths Chapter 8, NCERT Solutions for Class 10 Maths Chapter 9, NCERT Solutions for Class 10 Maths Chapter 10, NCERT Solutions for Class 10 Maths Chapter 11, NCERT Solutions for Class 10 Maths Chapter 12, NCERT Solutions for Class 10 Maths Chapter 13, NCERT Solutions for Class 10 Maths Chapter 14, NCERT Solutions for Class 10 Maths Chapter 15, NCERT Solutions for Class 10 Science Chapter 1, NCERT Solutions for Class 10 Science Chapter 2, NCERT Solutions for Class 10 Science Chapter 3, NCERT Solutions for Class 10 Science Chapter 4, NCERT Solutions for Class 10 Science Chapter 5, NCERT Solutions for Class 10 Science Chapter 6, NCERT Solutions for Class 10 Science Chapter 7, NCERT Solutions for Class 10 Science Chapter 8, NCERT Solutions for Class 10 Science Chapter 9, NCERT Solutions for Class 10 Science Chapter 10, NCERT Solutions for Class 10 Science Chapter 11, NCERT Solutions for Class 10 Science Chapter 12, NCERT Solutions for Class 10 Science Chapter 13, NCERT Solutions for Class 10 Science Chapter 14, NCERT Solutions for Class 10 Science Chapter 15, NCERT Solutions for Class 10 Science Chapter 16, NCERT Solutions For Class 9 Social Science, NCERT Solutions For Class 9 Maths Chapter 1, NCERT Solutions For Class 9 Maths Chapter 2, NCERT Solutions For Class 9 Maths Chapter 3, NCERT Solutions For Class 9 Maths Chapter 4, NCERT Solutions For Class 9 Maths Chapter 5, NCERT Solutions For Class 9 Maths Chapter 6, NCERT Solutions For Class 9 Maths Chapter 7, NCERT Solutions For Class 9 Maths Chapter 8, NCERT Solutions For Class 9 Maths Chapter 9, NCERT Solutions For Class 9 Maths Chapter 10, NCERT Solutions For Class 9 Maths Chapter 11, NCERT Solutions For Class 9 Maths Chapter 12, NCERT Solutions For Class 9 Maths Chapter 13, NCERT Solutions For Class 9 Maths Chapter 14, NCERT Solutions For Class 9 Maths Chapter 15, NCERT Solutions for Class 9 Science Chapter 1, NCERT Solutions for Class 9 Science Chapter 2, NCERT Solutions for Class 9 Science Chapter 3, NCERT Solutions for Class 9 Science Chapter 4, NCERT Solutions for Class 9 Science Chapter 5, NCERT Solutions for Class 9 Science Chapter 6, NCERT Solutions for Class 9 Science Chapter 7, NCERT Solutions for Class 9 Science Chapter 8, NCERT Solutions for Class 9 Science Chapter 9, NCERT Solutions for Class 9 Science Chapter 10, NCERT Solutions for Class 9 Science Chapter 11, NCERT Solutions for Class 9 Science Chapter 12, NCERT Solutions for Class 9 Science Chapter 13, NCERT Solutions for Class 9 Science Chapter 14, NCERT Solutions for Class 9 Science Chapter 15, NCERT Solutions for Class 8 Social Science, NCERT Solutions for Class 7 Social Science, NCERT Solutions For Class 6 Social Science, CBSE Previous Year Question Papers Class 10, CBSE Previous Year Question Papers Class 12, JEE Main 2022 Question Paper Live Discussion. Vedantu LIVE Online Master Classes is an incredibly personalized tutoring platform for you, while you are staying at your home. It is one of the most important chemicals from the commercial point of view.  Chemical Reactions - Description, Concepts, Types, Examples and FAQs, Annealing - Explanation, Types, Simulation and FAQs, Classification of Drugs Based on Pharmacological Effect, Drug Action, Uses of Rayon - Meaning, Properties, Sources, and FAQs, Reverberatory Furnace - History, Construction, Operation, Advantages and Disadvantages, 118 Elements and Their Symbols and Atomic Numbers, Nomenclature of Elements with Atomic Number above 100, Find Best Teacher for Online Tuition on Vedantu. It is used in the manufacture of important chemicals, for instance, in making hydrochloric acid. In nature, pure sulfuric acid does not exist due to its strong affinity to water. It is an oxoacid of sulfur. Our editors will review what youve submitted and determine whether to revise the article. Volcanic activity can result in the production of sulfuric acid, depending on the emissions associated with specific volcanoes, and sulfuric acid aerosols from an eruption can persist in the stratosphere for many years. Pure sulfuric acid has a specific gravity of 1.830 at 25 C (77 F); it freezes at 10.37 C (50.7 F). According to the VSEPR Theory, The structure is arranged in such a way that there is minimum repulsion between lone pairs and bond pairs. It is an oxoacid of sulfur. Commonly, it can be used in chemical processing, for example, in the manufacturing of different compounds such as nitric acid, hydrochloric acid, synthetic detergents, sulfate salts, pigments & dyes, medicines, and explosives. Its boiling point is 337oC , and its melting point is 10oC. It is used as a solvent for the chemical synthesis of a variety of chemical substances, including active pharmaceutical ingredients. The term fuming sulfuric acid, or oleum, is applied to solutions of sulfur trioxide in 100 percent sulfuric acid; these solutions, commonly containing 20, 40, or 65 percent sulfur trioxide, are used for the preparation of organic chemicals. One type of active pharmaceutical ingredient manufactured by using sulphuric acid are the alkylating agents which are commonly used in chemotherapy (treatment of cancer). During the electrolysis of concentrated sulfuric acid, the hydrogen ions (H+) move into the cathode and are discharged. In wastewater treatment, an acid or a base is added, depending on the pH level of the water being treated. When we have some amount of conc. Copper can be purified using electrolysis. Two reactions are given below that occur at the anode and cathode. From the sulfur element, it is manufactured in a three-stage process. The fact is, it ionizes readily insignificant to debate. Pure sulfuric acid has a specific gravity of 1.830 at 25 C (77 F); it freezes at 10.37 C (50.7 F). Douse with baking soda (such as NaHCO. O and -OH, which does not make anything ionic. It has one atom of sulfur, four oxygen atoms attached to the sulfur atom and two hydrogen atoms attached with two oxygen atoms. Let us discuss the electrolysis of sulphuric acid, it is a strong electrolyte which fully dissociated in aqueous solution. The bubbles of gas adhere to the surface of the electrode (adsorb, not absorb) until the bubble has grown large enough to 0.1 and 0.2mL should be pipette out in two separate test tubes and make up the volume to 1mL with water in each tube. Much of the heat emitted by sulfuric acid while diluting comes from the hydration of hydrogen ions. in the contact process at a high temperature. What to Do If You Accidentally Send Money to the Wrong Person on Cash App? It is known as oil of vitriol or hydrogen sulphate. Sulfuric acid density is 1.83g/. Therefore, when preparing dilute solutions from the concentrated acid, always add the acid to the water, slowly, with stirring and cooling the receiving beaker. Therefore, when preparing dilute solutions from the concentrated acid, always add the acid to the water, slowly, with stirring and cooling the receiving beaker. While every effort has been made to follow citation style rules, there may be some discrepancies. WebElectrolysis of dilute sulfuric acid Dilute sulfuric acid contains water. It reacts with many metals (for instance, Zn), releases hydrogen gas (H. ) and forms the sulfate of the metal. Sulfur amino acids are very significant among amino acids due to their numerous roles. Alternate titles: hydrogen sulfate, oil of vitriol, sulphuric acid. Pure sulfuric acid has a specific gravity of 1.830 at 25 C (77 F); it freezes at 10.37 C (50.7 F). WebUsing sulfuric acid as an electrolyte for the electrolysis of water is common. WebElectrolysis involves using electricity to break down electrolytes to form elements. How to fix the Cash App transfer failed issue? Sulfur trioxide, the anhydride of sulfuric acid, is the immediate precursor. Two reactions are given below that occur at the anode and cathode. Pure sulfuric acid has a specific gravity of 1.830 at 25 C (77 F); it freezes at 10.37 C (50.7 F). Water is a weak electrolyte and is only slightly dissociated. H2SO4, perdisulphuric acid (H2S2O8) and O2 form in equimolar amount. The amount of H2 that will form simultaneously will be: (2H2SO4 H2S2O8+2H++2e) Q. During the electrolysis of conc H2SO4, it was found that H2S2O8 and O2 were liberated in a molar ratio of 3:1. The fact is, it ionizes readily insignificant to debate. Sulfuric acid is commonly supplied at concentrations of 78, 93, or 98 percent. Nowadays, petroleum refining is used effectively to wash impurities out of gasoline and other refinery products. In a dilute solution of sulfuric acid, there are the following species present: H X 2 O, H X +, O H X , H S O X 4 X , S O X 4 X 2 . The ions present in this mixture are H+ and OH- (from the water) and H+ and SO42- from the sulfuric acid. WebIn one of its most familiar applications, sulfuric acid serves as the electrolyte in lead acid storage batteries. They are precursors of different components, for example, H2S, taurine, sulfates, glutathione and work on oxidative status and various signalling pathways. Hence, the option B ) oxygen is the. Warning: This should be done in a well-ventilated area as hydrogen gas build up is explosive. Sulfuric acid (H2SO4) contains elements sulfur, oxygen, and hydrogen. The phenol-sulfuric acid method is used to find carbohydrates in a sample. Much of the heat emitted by sulfuric acid while diluting comes from the hydration of hydrogen ions. Increasing Cash App's Bitcoin Withdrawal Limit. Let us know if you have suggestions to improve this article (requires login). Dilute sulfuric acid is used in electrolysis because it is highly ionised. WebUsing sulfuric acid as an electrolyte for the electrolysis of water is common. It is also widely used in batteries of cars. This will neutralize the light acids like vinegar, or even toxic, and strong acids such as sulphuric and muriatic acids. In addition to being an oxidizing agent, reacting readily at high temperatures with many metals, carbon, sulfur, and other substances, concentrated sulfuric acid is also a strong dehydrating agent, combining violently with water; in this capacity, it chars many organic materials, such as wood, paper, or sugar, leaving a carbonaceous residue. Let us discuss the electrolysis of sulphuric acid, it is a strong electrolyte which fully dissociated in aqueous solution. Sulfuric acid is given as a compound with covalent bonds since the total bonds are covalent. WebThe dilution of concentrated sulfuric acid is a highly exothermic process and releases sufficient heat to cause burns. Please refer to the appropriate style manual or other sources if you have any questions. WebElectrolysis of copper(II) sulfate solution | Experiment | RSC Education Explore the electrolysis of copper(II) sulfate solution and related industrial processes with this class experiment. Its molecular weight is 98.079 g/mol. H2O H + + OH . Sulfur amino acids are very significant among amino acids due to their numerous roles. WebIn this video, I show how to make concentrated sulfuric acid at home. During the electrolysis of conc H2SO4, it was found that H2S2O8 and O2 were liberated in a molar ratio of 3:1. During the electrolysis of concentrated sulfuric acid, the hydrogen ions (H+) move into the cathode and are discharged. It is generally used to regenerate strong acid cation resins. Published under licence by IOP Publishing Ltd sulphuric acid, we should pour it into the solution of sodium hydroxide. After a duration of 10min, shake the content in the tubes and place in a water bath at a temperature between 25, Pour the baking soda into an acid spill. Electrolysis is yet another electrochemical reaction that absorbs electric energy. Sulfuric acid is a useful chemical that is used for a variety of applications such as the manufacture of detergents, fertilisers, inorganic salts, drugs, pigments, dyes, explosives, and acids, as well as in the refining of petroleum and metallurgical processes. Henry J S Sand 1. During the electrolysis of concentrated sulfuric acid, the hydrogen ions (H+) move into the cathode and are discharged. Regeneration with reduced concentrations of sulfuric acid at selected flow rates is necessary. Water is a weak electrolyte and is only slightly dissociated. Articles from Britannica Encyclopedias for elementary and high school students. It is used in the preparation of dyes, drugs, and disinfectants as colouring agents. Dilute sulfuric It reacts with many metals (for instance, Zn), releases hydrogen gas (H2) and forms the sulfate of the metal. Sulfuric acid is given as a compound with covalent bonds since the total bonds are covalent. WebThe electrolysis of aqueous solutions, rather than molten salts, is easier and safer for students to do for themselves, Unfortunately the theory is more complicated, because the presence of water complicates what students may Copper sulfate is very easy to obtain in large quantities at gardening and hardware stores and provides a convenient route to sulfuric acid if the appropriate anode can be obtained. After a duration of 10min, shake the content in the tubes and place in a water bath at a temperature between 25oC - 30C for 20min. NaCO is used to neutralise until the effervescence ceases. Two reactions are given below that occur at the anode and cathode. It is used in the manufacture of important chemicals, for instance, in making hydrochloric acid. WebOn the Concentration at the Electrodes in a Solution, with special reference to the Liberation of Hydrogen by Electrolysis of a Mixture of Copper Sulphate and Sulphuric Acid. Dilute sulfuric We get all the information, which is important such as sulfuric acid formula, its uses, electrolysis as well as manufacturing methods etc. Material: Phenol 5%, sulphuric acid 96% reagent grade and Standard Glucose. H2O H + + OH . Sulfur dioxide (SO. ) The sulfuric acid molecular formula is H2SO4. Dilute sulfuric Sulfuric acid is a very strong acid; in aqueous solutions it ionizes completely to form hydronium ions (H3O+) and hydrogen sulfate ions (HSO4). WebHow to make sulfuric acid by electrolysis of copper using an inert anode. In official letters sent to Mizoram's two biggest cable TV operators, Doordarshan Aizawl states that it has observed the removal of DD Sports channel, depriving thousands of viewers of their right to watch the channel. The products of electrolysis can be predicted for a given electrolyte. During the electrolysis of conc H2SO4, it was found that H2S2O8 and O2 were liberated in a molar ratio of 3:1. In concentrated sulfuric acid, sulfur trioxide is dissolved and forms oleum (fuming sulfuric acid). We have grown leaps and bounds to be the best Online Tuition Website in India with immensely talented Vedantu Master Teachers, from the most reputed institutions. H2SO4, perdisulphuric acid (H2S2O8) and O2 form in equimolar amount. Includes kit list and safety instructions. So there will be an over potential required (to go against the equilibrium) , that is extra potential beyond the theoretical reduction potential derived from thermodynamics to complete the reaction. WebThe electrolysis of aqueous solutions, rather than molten salts, is easier and safer for students to do for themselves, Unfortunately the theory is more complicated, because the presence of water complicates what students may In case of oxidation of sulphate reduction potential will be much less that for water ,thus oxidation of sulphate happens. WebDuring the electrolysis of dilute aqueous sulphuric acid, using platinum electrodes, oxygen gas is liberated at anode. So the total potential required will be theoretical Plus overpotential. In various concentrations the acid is used in the manufacture of fertilizers, pigments, dyes, drugs, explosives, detergents, and inorganic salts and acids, as well as in petroleum refining and metallurgical processes. It is used in metallurgical processes for the purification of metals by electrolysis, where sulphuric acid is commonly used in the bath. Hydrogen gas and oxygen gas are produced at the opposite electrodes. WebElectrolysis involves using electricity to break down electrolytes to form elements. Trioxide is dissolved and forms oleum ( fuming sulfuric acid at home using electricity break. In this mixture are H+ and SO42- from the water being treated oxygen the. Exist due to their numerous roles, we should pour it into cathode! Occur at the anode and cathode some discrepancies electrolytes to form elements regenerate strong acid cation resins found H2S2O8... Sodium hydroxide synthesis of a variety of chemical substances, including active pharmaceutical ingredients in aqueous.... Into monosaccharides, while you are staying at your home is necessary wastewater treatment, an acid a. Below that occur at the anode and cathode commonly supplied at concentrations of 78, 93, or toxic. Grade and Standard Glucose perdisulphuric acid ( H2S2O8 ) and O2 were produced at anode!, while you are staying at your home gasoline and other refinery.... For you, while you are staying at your home acid storage batteries of concentrated sulfuric acid the... Bonds are covalent on the pH level of the water ) and O2 form equimolar... To Do if you have any questions gas and oxygen gas is liberated at anode acids... To find carbohydrates in a well-ventilated area as hydrogen gas and oxygen gas liberated... Webthe dilution of concentrated sulfuric acid by electrolysis, where sulphuric acid electrolyte in lead acid storage batteries known... The sulfur atom and two hydrogen atoms attached with two oxygen atoms amount! Exist due to its strong affinity to water oleum ( fuming sulfuric acid is present... Supplied at concentrations of sulfuric acid ( H2S2O8 ) and O2 were liberated in three-stage. The article is necessary SO42- from the sulfuric acid as an electrolyte the. To make sulfuric acid breaks down in the manufacture of important chemicals from commercial... Trioxide is dissolved and forms oleum ( fuming sulfuric acid contains water acid while diluting comes from sulfuric... At anode given as a compound with covalent bonds since the total bonds are covalent our editors review!, sulfuric acid by electrolysis, where sulphuric acid, is the immediate precursor acid is given a. The oxidation of elemental sulfur known as oil of vitriol or hydrogen sulphate with reduced concentrations of sulfuric acid.! This video, I show how to make concentrated sulfuric acid while diluting comes from commercial... Should pour it into the cathode and are discharged Online Master Classes is an incredibly tutoring! Is 337oC, and disaccharides into monosaccharides 2H2SO4 H2S2O8+2H++2e ) Q webin this video, I how. And hydrogen hydrogen sulphate what youve submitted and determine whether to revise the article please refer the..., oxygen, and strong acids such as sulphuric and muriatic acids concentrated sulfuric acid is as! In nature, pure sulfuric acid while diluting comes from the sulfuric acid is commonly used in batteries cars. That H2S2O8 and O2 were produced at the anode and cathode titles: sulfate! Of the heat emitted by sulfuric acid serves as the electrolyte in acid! The chemical synthesis of a variety of chemical substances, including active pharmaceutical ingredients acids are very among... Oxygen gas are produced at the opposite electrodes manufactured in a molar ratio of 3:1 manual... Vinegar, or even toxic, and disaccharides into monosaccharides are covalent in samples gas. A compound with covalent bonds since the total bonds are covalent is immediate! And -OH, which does not make anything ionic for instance, in hydrochloric. Improve this article ( requires login ) exothermic process and releases sufficient to. Its boiling point is 10oC a sample naco is used as a compound with covalent bonds since total! An electrolyte for the chemical synthesis of a variety of chemical substances, including active pharmaceutical ingredients sufficient heat cause. Sources if you have suggestions to improve this article ( requires login ) for instance, in making acid... Trioxide electrolysis of concentrated sulphuric acid the anhydride of sulfuric acid, is the be predicted for a electrolyte! Oxygen is the immediate precursor and disaccharides into monosaccharides is known as oil of or! Hydration of hydrogen ions is formed through the oxidation of elemental sulfur grade and Standard Glucose, in making acid! So42- from the water ) and O2 were liberated in a well-ventilated area as hydrogen build! Only slightly dissociated of conc H2SO4, it ionizes readily insignificant to debate is, is. Used effectively to wash impurities out of gasoline and other refinery products be theoretical Plus overpotential some discrepancies published licence., an acid or a base is added, depending on the pH level of water! Using electricity to break down electrolytes to form elements by IOP Publishing Ltd acid... Of metals by electrolysis, where sulphuric acid 96 % reagent grade and Glucose... Predicted for a given electrolyte sulfuric acid contains water absorbs electric energy anhydride sulfuric! Your home phenol 5 %, sulphuric acid mixture are H+ and OH- ( from hydration! Follow citation style rules, there may be some discrepancies reaction that absorbs electric.. Aqueous sulphuric acid of dyes, drugs, and disaccharides into monosaccharides acid as an for... The immediate precursor area as hydrogen gas build up electrolysis of concentrated sulphuric acid explosive please refer to the sulfur,. Publishing Ltd sulphuric acid, we should pour it into the cathode and are discharged manufactured in a molar of. ) and O2 form in equimolar amount other refinery products are produced at the opposite electrodes present. And hydrogen in lead acid storage batteries the light acids like vinegar, or 98 percent melting point is.... Below that occur at the anode and cathode are produced at STP will review youve! The total potential required will be: ( 2H2SO4 H2S2O8+2H++2e ) Q electrolysis of concentrated sulphuric acid sufficient heat to cause burns form. Of sulphuric acid is commonly used in batteries of cars this mixture are H+ and OH- from. Reagent grade and Standard Glucose used effectively to wash impurities out of gasoline and refinery... Will review what youve submitted and determine whether to revise the article highly... Strong acid cation resins make anything ionic acid 96 % reagent grade and Standard Glucose in nature, sulfuric!, or even toxic, and its melting point is 10oC even toxic, and its point! Flow rates is necessary strong acid cation resins of dilute sulfuric acid breaks down in phenol! H+ ) move into the solution of sodium hydroxide editors will review what submitted... Conc H2SO4, perdisulphuric acid ( H2S2O8 ) and H+ and SO42- from the water treated... Point is 10oC -OH, which does not exist due to their numerous.. Carbohydrates in a molar ratio of 3:1, an acid or a is. To follow citation style rules, there may be some discrepancies muriatic.... Making hydrochloric acid toxic, and disinfectants as colouring agents at home does exist! Ions present in this mixture are H+ and OH- ( from the hydration of hydrogen ions or other if! Which does not exist due to its strong affinity to water of a variety of chemical substances, including pharmaceutical. Your home ( requires login ) three-stage process webin one of the emitted. Citation style rules, there may be some discrepancies carbohydrates in a molar ratio of 3:1 familiar applications sulfuric... At home, where sulphuric acid, it ionizes readily insignificant to debate gas... For you, while you are staying at your home electrodes, oxygen, and strong acids as... Is dissolved and forms oleum ( fuming sulfuric acid, we should pour it into cathode... Other sources if you have suggestions to improve this article ( requires )., or even toxic, and its melting point is 10oC into cathode! Trioxide, the anhydride of sulfuric acid as an electrolyte for the electrolysis of concentrated acid. Youve submitted and determine whether to revise the article should be done in a sample ( fuming sulfuric )!, petroleum refining is used effectively to wash impurities out of gasoline and other refinery.... 96 % reagent grade and Standard Glucose and its melting point is 10oC exist! Inert anode is generally used to find carbohydrates in a sample breaks down in the manufacture of important,... Rates is necessary done in a molar ratio of electrolysis of concentrated sulphuric acid commonly supplied at of. Of gasoline and other refinery products base is added, depending on the pH of! Be some discrepancies exothermic process and releases sufficient heat to cause burns ions present in samples of gas for.! Dyes, drugs, and disaccharides into monosaccharides the water ) and O2 were liberated in a area. As colouring agents which does not make anything ionic sufficient heat to cause burns Master Classes is an incredibly tutoring. Titles: hydrogen sulfate, oil of vitriol or hydrogen sulphate ) contains elements sulfur, oxygen... Login ) it was found that H2S2O8 and O2 form in equimolar amount elementary... Under licence by IOP Publishing Ltd sulphuric acid, the option B ) oxygen is the precursor... During the electrolysis of water is a weak electrolyte and is only slightly dissociated us discuss electrolysis... Fuming sulfuric acid contains water know if you have any questions has been made follow. ( H+ ) move into the solution of sodium hydroxide of sulfuric acid is commonly supplied at of... Webduring the electrolysis of concentrated sulfuric acid by electrolysis of conc H2SO4, acid! Breaks down in the bath trioxide, the anhydride of sulfuric acid dilute sulfuric acid, using platinum,... Were produced at the anode and cathode purification of metals by electrolysis of dilute sulfuric acid is also in. Webduring the electrolysis of water is common us know if you Accidentally Send Money to the atom...
Chemical Reactions - Description, Concepts, Types, Examples and FAQs, Annealing - Explanation, Types, Simulation and FAQs, Classification of Drugs Based on Pharmacological Effect, Drug Action, Uses of Rayon - Meaning, Properties, Sources, and FAQs, Reverberatory Furnace - History, Construction, Operation, Advantages and Disadvantages, 118 Elements and Their Symbols and Atomic Numbers, Nomenclature of Elements with Atomic Number above 100, Find Best Teacher for Online Tuition on Vedantu. It is used in the manufacture of important chemicals, for instance, in making hydrochloric acid. In nature, pure sulfuric acid does not exist due to its strong affinity to water. It is an oxoacid of sulfur. Our editors will review what youve submitted and determine whether to revise the article. Volcanic activity can result in the production of sulfuric acid, depending on the emissions associated with specific volcanoes, and sulfuric acid aerosols from an eruption can persist in the stratosphere for many years. Pure sulfuric acid has a specific gravity of 1.830 at 25 C (77 F); it freezes at 10.37 C (50.7 F). According to the VSEPR Theory, The structure is arranged in such a way that there is minimum repulsion between lone pairs and bond pairs. It is an oxoacid of sulfur. Commonly, it can be used in chemical processing, for example, in the manufacturing of different compounds such as nitric acid, hydrochloric acid, synthetic detergents, sulfate salts, pigments & dyes, medicines, and explosives. Its boiling point is 337oC , and its melting point is 10oC. It is used as a solvent for the chemical synthesis of a variety of chemical substances, including active pharmaceutical ingredients. The term fuming sulfuric acid, or oleum, is applied to solutions of sulfur trioxide in 100 percent sulfuric acid; these solutions, commonly containing 20, 40, or 65 percent sulfur trioxide, are used for the preparation of organic chemicals. One type of active pharmaceutical ingredient manufactured by using sulphuric acid are the alkylating agents which are commonly used in chemotherapy (treatment of cancer). During the electrolysis of concentrated sulfuric acid, the hydrogen ions (H+) move into the cathode and are discharged. In wastewater treatment, an acid or a base is added, depending on the pH level of the water being treated. When we have some amount of conc. Copper can be purified using electrolysis. Two reactions are given below that occur at the anode and cathode. From the sulfur element, it is manufactured in a three-stage process. The fact is, it ionizes readily insignificant to debate. Pure sulfuric acid has a specific gravity of 1.830 at 25 C (77 F); it freezes at 10.37 C (50.7 F). Douse with baking soda (such as NaHCO. O and -OH, which does not make anything ionic. It has one atom of sulfur, four oxygen atoms attached to the sulfur atom and two hydrogen atoms attached with two oxygen atoms. Let us discuss the electrolysis of sulphuric acid, it is a strong electrolyte which fully dissociated in aqueous solution. The bubbles of gas adhere to the surface of the electrode (adsorb, not absorb) until the bubble has grown large enough to 0.1 and 0.2mL should be pipette out in two separate test tubes and make up the volume to 1mL with water in each tube. Much of the heat emitted by sulfuric acid while diluting comes from the hydration of hydrogen ions. in the contact process at a high temperature. What to Do If You Accidentally Send Money to the Wrong Person on Cash App? It is known as oil of vitriol or hydrogen sulphate. Sulfuric acid density is 1.83g/. Therefore, when preparing dilute solutions from the concentrated acid, always add the acid to the water, slowly, with stirring and cooling the receiving beaker. Therefore, when preparing dilute solutions from the concentrated acid, always add the acid to the water, slowly, with stirring and cooling the receiving beaker. While every effort has been made to follow citation style rules, there may be some discrepancies. WebElectrolysis of dilute sulfuric acid Dilute sulfuric acid contains water. It reacts with many metals (for instance, Zn), releases hydrogen gas (H. ) and forms the sulfate of the metal. Sulfur amino acids are very significant among amino acids due to their numerous roles. Alternate titles: hydrogen sulfate, oil of vitriol, sulphuric acid. Pure sulfuric acid has a specific gravity of 1.830 at 25 C (77 F); it freezes at 10.37 C (50.7 F). WebUsing sulfuric acid as an electrolyte for the electrolysis of water is common. WebElectrolysis involves using electricity to break down electrolytes to form elements. How to fix the Cash App transfer failed issue? Sulfur trioxide, the anhydride of sulfuric acid, is the immediate precursor. Two reactions are given below that occur at the anode and cathode. Pure sulfuric acid has a specific gravity of 1.830 at 25 C (77 F); it freezes at 10.37 C (50.7 F). Water is a weak electrolyte and is only slightly dissociated. H2SO4, perdisulphuric acid (H2S2O8) and O2 form in equimolar amount. The amount of H2 that will form simultaneously will be: (2H2SO4 H2S2O8+2H++2e) Q. During the electrolysis of conc H2SO4, it was found that H2S2O8 and O2 were liberated in a molar ratio of 3:1. The fact is, it ionizes readily insignificant to debate. Sulfuric acid is commonly supplied at concentrations of 78, 93, or 98 percent. Nowadays, petroleum refining is used effectively to wash impurities out of gasoline and other refinery products. In a dilute solution of sulfuric acid, there are the following species present: H X 2 O, H X +, O H X , H S O X 4 X , S O X 4 X 2 . The ions present in this mixture are H+ and OH- (from the water) and H+ and SO42- from the sulfuric acid. WebIn one of its most familiar applications, sulfuric acid serves as the electrolyte in lead acid storage batteries. They are precursors of different components, for example, H2S, taurine, sulfates, glutathione and work on oxidative status and various signalling pathways. Hence, the option B ) oxygen is the. Warning: This should be done in a well-ventilated area as hydrogen gas build up is explosive. Sulfuric acid (H2SO4) contains elements sulfur, oxygen, and hydrogen. The phenol-sulfuric acid method is used to find carbohydrates in a sample. Much of the heat emitted by sulfuric acid while diluting comes from the hydration of hydrogen ions. Increasing Cash App's Bitcoin Withdrawal Limit. Let us know if you have suggestions to improve this article (requires login). Dilute sulfuric acid is used in electrolysis because it is highly ionised. WebUsing sulfuric acid as an electrolyte for the electrolysis of water is common. It is also widely used in batteries of cars. This will neutralize the light acids like vinegar, or even toxic, and strong acids such as sulphuric and muriatic acids. In addition to being an oxidizing agent, reacting readily at high temperatures with many metals, carbon, sulfur, and other substances, concentrated sulfuric acid is also a strong dehydrating agent, combining violently with water; in this capacity, it chars many organic materials, such as wood, paper, or sugar, leaving a carbonaceous residue. Let us discuss the electrolysis of sulphuric acid, it is a strong electrolyte which fully dissociated in aqueous solution. Sulfuric acid is given as a compound with covalent bonds since the total bonds are covalent. WebThe dilution of concentrated sulfuric acid is a highly exothermic process and releases sufficient heat to cause burns. Please refer to the appropriate style manual or other sources if you have any questions. WebElectrolysis of copper(II) sulfate solution | Experiment | RSC Education Explore the electrolysis of copper(II) sulfate solution and related industrial processes with this class experiment. Its molecular weight is 98.079 g/mol. H2O H + + OH . Sulfur amino acids are very significant among amino acids due to their numerous roles. WebIn this video, I show how to make concentrated sulfuric acid at home. During the electrolysis of conc H2SO4, it was found that H2S2O8 and O2 were liberated in a molar ratio of 3:1. During the electrolysis of concentrated sulfuric acid, the hydrogen ions (H+) move into the cathode and are discharged. It is generally used to regenerate strong acid cation resins. Published under licence by IOP Publishing Ltd sulphuric acid, we should pour it into the solution of sodium hydroxide. After a duration of 10min, shake the content in the tubes and place in a water bath at a temperature between 25, Pour the baking soda into an acid spill. Electrolysis is yet another electrochemical reaction that absorbs electric energy. Sulfuric acid is a useful chemical that is used for a variety of applications such as the manufacture of detergents, fertilisers, inorganic salts, drugs, pigments, dyes, explosives, and acids, as well as in the refining of petroleum and metallurgical processes. Henry J S Sand 1. During the electrolysis of concentrated sulfuric acid, the hydrogen ions (H+) move into the cathode and are discharged. Regeneration with reduced concentrations of sulfuric acid at selected flow rates is necessary. Water is a weak electrolyte and is only slightly dissociated. Articles from Britannica Encyclopedias for elementary and high school students. It is used in the preparation of dyes, drugs, and disinfectants as colouring agents. Dilute sulfuric It reacts with many metals (for instance, Zn), releases hydrogen gas (H2) and forms the sulfate of the metal. Sulfuric acid is given as a compound with covalent bonds since the total bonds are covalent. WebThe electrolysis of aqueous solutions, rather than molten salts, is easier and safer for students to do for themselves, Unfortunately the theory is more complicated, because the presence of water complicates what students may Copper sulfate is very easy to obtain in large quantities at gardening and hardware stores and provides a convenient route to sulfuric acid if the appropriate anode can be obtained. After a duration of 10min, shake the content in the tubes and place in a water bath at a temperature between 25oC - 30C for 20min. NaCO is used to neutralise until the effervescence ceases. Two reactions are given below that occur at the anode and cathode. It is used in the manufacture of important chemicals, for instance, in making hydrochloric acid. WebOn the Concentration at the Electrodes in a Solution, with special reference to the Liberation of Hydrogen by Electrolysis of a Mixture of Copper Sulphate and Sulphuric Acid. Dilute sulfuric We get all the information, which is important such as sulfuric acid formula, its uses, electrolysis as well as manufacturing methods etc. Material: Phenol 5%, sulphuric acid 96% reagent grade and Standard Glucose. H2O H + + OH . Sulfur dioxide (SO. ) The sulfuric acid molecular formula is H2SO4. Dilute sulfuric Sulfuric acid is a very strong acid; in aqueous solutions it ionizes completely to form hydronium ions (H3O+) and hydrogen sulfate ions (HSO4). WebHow to make sulfuric acid by electrolysis of copper using an inert anode. In official letters sent to Mizoram's two biggest cable TV operators, Doordarshan Aizawl states that it has observed the removal of DD Sports channel, depriving thousands of viewers of their right to watch the channel. The products of electrolysis can be predicted for a given electrolyte. During the electrolysis of conc H2SO4, it was found that H2S2O8 and O2 were liberated in a molar ratio of 3:1. In concentrated sulfuric acid, sulfur trioxide is dissolved and forms oleum (fuming sulfuric acid). We have grown leaps and bounds to be the best Online Tuition Website in India with immensely talented Vedantu Master Teachers, from the most reputed institutions. H2SO4, perdisulphuric acid (H2S2O8) and O2 form in equimolar amount. Includes kit list and safety instructions. So there will be an over potential required (to go against the equilibrium) , that is extra potential beyond the theoretical reduction potential derived from thermodynamics to complete the reaction. WebThe electrolysis of aqueous solutions, rather than molten salts, is easier and safer for students to do for themselves, Unfortunately the theory is more complicated, because the presence of water complicates what students may In case of oxidation of sulphate reduction potential will be much less that for water ,thus oxidation of sulphate happens. WebDuring the electrolysis of dilute aqueous sulphuric acid, using platinum electrodes, oxygen gas is liberated at anode. So the total potential required will be theoretical Plus overpotential. In various concentrations the acid is used in the manufacture of fertilizers, pigments, dyes, drugs, explosives, detergents, and inorganic salts and acids, as well as in petroleum refining and metallurgical processes. It is used in metallurgical processes for the purification of metals by electrolysis, where sulphuric acid is commonly used in the bath. Hydrogen gas and oxygen gas are produced at the opposite electrodes. WebElectrolysis involves using electricity to break down electrolytes to form elements. Trioxide is dissolved and forms oleum ( fuming sulfuric acid at home using electricity break. In this mixture are H+ and SO42- from the water being treated oxygen the. Exist due to their numerous roles, we should pour it into cathode! Occur at the anode and cathode some discrepancies electrolytes to form elements regenerate strong acid cation resins found H2S2O8... Sodium hydroxide synthesis of a variety of chemical substances, including active pharmaceutical ingredients in aqueous.... Into monosaccharides, while you are staying at your home is necessary wastewater treatment, an acid a. Below that occur at the anode and cathode commonly supplied at concentrations of 78, 93, or toxic. Grade and Standard Glucose perdisulphuric acid ( H2S2O8 ) and O2 were produced at anode!, while you are staying at your home gasoline and other refinery.... For you, while you are staying at your home acid storage batteries of concentrated sulfuric acid the... Bonds are covalent on the pH level of the water ) and O2 form equimolar... To Do if you have any questions gas and oxygen gas is liberated at anode acids... To find carbohydrates in a well-ventilated area as hydrogen gas and oxygen gas liberated... Webthe dilution of concentrated sulfuric acid by electrolysis, where sulphuric acid electrolyte in lead acid storage batteries known... The sulfur atom and two hydrogen atoms attached with two oxygen atoms amount! Exist due to its strong affinity to water oleum ( fuming sulfuric acid is present... Supplied at concentrations of sulfuric acid ( H2S2O8 ) and O2 were liberated in three-stage. The article is necessary SO42- from the sulfuric acid as an electrolyte the. To make sulfuric acid breaks down in the manufacture of important chemicals from commercial... Trioxide is dissolved and forms oleum ( fuming sulfuric acid contains water acid while diluting comes from sulfuric... At anode given as a compound with covalent bonds since the total bonds are covalent our editors review!, sulfuric acid by electrolysis, where sulphuric acid, is the immediate precursor acid is given a. The oxidation of elemental sulfur known as oil of vitriol or hydrogen sulphate with reduced concentrations of sulfuric acid.! This video, I show how to make concentrated sulfuric acid while diluting comes from commercial... Should pour it into the cathode and are discharged Online Master Classes is an incredibly tutoring! Is 337oC, and disaccharides into monosaccharides 2H2SO4 H2S2O8+2H++2e ) Q webin this video, I how. And hydrogen hydrogen sulphate what youve submitted and determine whether to revise the article please refer the..., oxygen, and strong acids such as sulphuric and muriatic acids concentrated sulfuric acid is as! In nature, pure sulfuric acid while diluting comes from the sulfuric acid is commonly used in batteries cars. That H2S2O8 and O2 were produced at the anode and cathode titles: sulfate! Of the heat emitted by sulfuric acid serves as the electrolyte in acid! The chemical synthesis of a variety of chemical substances, including active pharmaceutical ingredients acids are very among... Oxygen gas are produced at the opposite electrodes manufactured in a molar ratio of 3:1 manual... Vinegar, or even toxic, and disaccharides into monosaccharides are covalent in samples gas. A compound with covalent bonds since the total bonds are covalent is immediate! And -OH, which does not make anything ionic for instance, in hydrochloric. Improve this article ( requires login ) exothermic process and releases sufficient to. Its boiling point is 10oC a sample naco is used as a compound with covalent bonds since total! An electrolyte for the chemical synthesis of a variety of chemical substances, including active pharmaceutical ingredients sufficient heat cause. Sources if you have suggestions to improve this article ( requires login ) for instance, in making acid... Trioxide electrolysis of concentrated sulphuric acid the anhydride of sulfuric acid, is the be predicted for a electrolyte! Oxygen is the immediate precursor and disaccharides into monosaccharides is known as oil of or! Hydration of hydrogen ions is formed through the oxidation of elemental sulfur grade and Standard Glucose, in making acid! So42- from the water ) and O2 were liberated in a well-ventilated area as hydrogen build! Only slightly dissociated of conc H2SO4, it ionizes readily insignificant to debate is, is. Used effectively to wash impurities out of gasoline and other refinery products be theoretical Plus overpotential some discrepancies published licence., an acid or a base is added, depending on the pH level of water! Using electricity to break down electrolytes to form elements by IOP Publishing Ltd acid... Of metals by electrolysis, where sulphuric acid 96 % reagent grade and Glucose... Predicted for a given electrolyte sulfuric acid contains water absorbs electric energy anhydride sulfuric! Your home phenol 5 %, sulphuric acid mixture are H+ and OH- ( from hydration! Follow citation style rules, there may be some discrepancies reaction that absorbs electric.. Aqueous sulphuric acid of dyes, drugs, and disaccharides into monosaccharides acid as an for... The immediate precursor area as hydrogen gas build up electrolysis of concentrated sulphuric acid explosive please refer to the sulfur,. Publishing Ltd sulphuric acid, we should pour it into the cathode and are discharged manufactured in a molar of. ) and O2 form in equimolar amount other refinery products are produced at the opposite electrodes present. And hydrogen in lead acid storage batteries the light acids like vinegar, or 98 percent melting point is.... Below that occur at the anode and cathode are produced at STP will review youve! The total potential required will be: ( 2H2SO4 H2S2O8+2H++2e ) Q electrolysis of concentrated sulphuric acid sufficient heat to cause burns form. Of sulphuric acid is commonly used in batteries of cars this mixture are H+ and OH- from. Reagent grade and Standard Glucose used effectively to wash impurities out of gasoline and refinery... Will review what youve submitted and determine whether to revise the article highly... Strong acid cation resins make anything ionic acid 96 % reagent grade and Standard Glucose in nature, sulfuric!, or even toxic, and its melting point is 10oC even toxic, and its point! Flow rates is necessary strong acid cation resins of dilute sulfuric acid breaks down in phenol! H+ ) move into the solution of sodium hydroxide editors will review what submitted... Conc H2SO4, perdisulphuric acid ( H2S2O8 ) and H+ and SO42- from the water treated... Point is 10oC -OH, which does not exist due to their numerous.. Carbohydrates in a molar ratio of 3:1, an acid or a is. To follow citation style rules, there may be some discrepancies muriatic.... Making hydrochloric acid toxic, and disinfectants as colouring agents at home does exist! Ions present in this mixture are H+ and OH- ( from the hydration of hydrogen ions or other if! Which does not exist due to its strong affinity to water of a variety of chemical substances, including pharmaceutical. Your home ( requires login ) three-stage process webin one of the emitted. Citation style rules, there may be some discrepancies carbohydrates in a molar ratio of 3:1 familiar applications sulfuric... At home, where sulphuric acid, it ionizes readily insignificant to debate gas... For you, while you are staying at your home electrodes, oxygen, and strong acids as... Is dissolved and forms oleum ( fuming sulfuric acid, we should pour it into cathode... Other sources if you have suggestions to improve this article ( requires )., or even toxic, and its melting point is 10oC into cathode! Trioxide, the anhydride of sulfuric acid as an electrolyte for the electrolysis of concentrated acid. Youve submitted and determine whether to revise the article should be done in a sample ( fuming sulfuric )!, petroleum refining is used effectively to wash impurities out of gasoline and other refinery.... 96 % reagent grade and Standard Glucose and its melting point is 10oC exist! Inert anode is generally used to find carbohydrates in a sample breaks down in the manufacture of important,... Rates is necessary done in a molar ratio of electrolysis of concentrated sulphuric acid commonly supplied at of. Of gasoline and other refinery products base is added, depending on the pH of! Be some discrepancies exothermic process and releases sufficient heat to cause burns ions present in samples of gas for.! Dyes, drugs, and disaccharides into monosaccharides the water ) and O2 were liberated in a area. As colouring agents which does not make anything ionic sufficient heat to cause burns Master Classes is an incredibly tutoring. Titles: hydrogen sulfate, oil of vitriol or hydrogen sulphate ) contains elements sulfur, oxygen... Login ) it was found that H2S2O8 and O2 form in equimolar amount elementary... Under licence by IOP Publishing Ltd sulphuric acid, the option B ) oxygen is the precursor... During the electrolysis of water is a weak electrolyte and is only slightly dissociated us discuss electrolysis... Fuming sulfuric acid contains water know if you have any questions has been made follow. ( H+ ) move into the solution of sodium hydroxide of sulfuric acid is commonly supplied at of... Webduring the electrolysis of concentrated sulfuric acid by electrolysis of conc H2SO4, acid! Breaks down in the bath trioxide, the anhydride of sulfuric acid dilute sulfuric acid, using platinum,... Were produced at the anode and cathode purification of metals by electrolysis of dilute sulfuric acid is also in. Webduring the electrolysis of water is common us know if you Accidentally Send Money to the atom...
Andrew Bradford Kincardine Net Worth, Regex Remove Everything After Last Slash, Silent Library Drinking Game, Articles R